Paramedics bring in a disheveled-appearing, anxious man who appears to be in his 20s. He was found wandering through traffic, muttering incoherently. As you approach him to introduce yourself, he becomes agitated. Eyes darting to and fro, he screams, “The devil is coming!” He leaps off the cot as paramedics frantically struggle to restrain him.
Does this scenario sound familiar?
Management of behavioral disturbance is a critical skill for emergency physicians. Nearly every shift we face agitated or psychotic patients who are suffering from psychiatric illness, drug abuse, or organic brain dysfunction. The goal is to quickly and safely calm down patients to prevent harm to themselves and the ED staff. Non-pharmacologic methods can be tried first, such as putting the patient in a dimly lit, quiet room, but pharmacologic agents are often needed. Haloperidol is one of the medications we first reach for, and here is everything you need to know to use it safely and effectively.
How It Works
Haloperidol, marketed under the brand name Haldol, is in the class of medications known as typical antipsychotics and has psychotropic, sedative, and antiemetic properties. While the precise mechanism of action is not known, its effects are believed to be mediated through blockade of postsynaptic dopaminergic D2 receptors in the brain. Haloperidol also has weak central anticholinergic effects. The antiemetic effects of haloperidol may be due to blockade of dopamine receptors in the chemoreceptor trigger zone. Other typical antipsychotics include chlorpromazine, droperidol, and fluphenazine [1,2].
Major Indications
Haloperidol is most commonly used in the ED for behavioral disturbance, to calm acutely psychotic or violent patients. Additionally, a low dose of haloperidol (0.5 mg) has been successfully used to help reduce anxiety or agitation of patients requiring BIPAP [3-5]. It is also used in a variety of other conditions, including Schizophrenia, Tourette’s syndrome, ICU delirium, and migraine headaches [6]. Haloperidol is a potent antiemetic, and has been successfully used to treat postoperative nausea and vomiting [2].
Notable History
Belgian scientists first synthesized Haloperidol in 1958 as part of an effort to create new analgesics. They quickly learned that while haloperidol did not have analgesic properties, it was a powerful neuroleptic. Within a decade haloperidol was widely used in the United States as an anti-psychotic and anti-hallucinogenic drug [7]. Today, haloperidol and other typical antipsychotics have largely been replaced for chronic treatment of psychiatric conditions by atypical antipsychotics such as risperidone, quetiapine, and olanzepine, which have lower rates of extrapyramidal symptoms and are often better tolerated.
Adverse Events
Cardiovascular: Haloperidol is associated with QT prolongation. In 2007, the U.S. Food and Drug administration issued an advisory warning on intravenous use of haloperidol, citing concerns that intravenous administration could trigger torsades de pointe and other ventricular arrhythmias. As such, haloperidol is not FDA approved for intravenous administration. However, subsequent studies have demonstrated that QT prolongation associated with haloperidol most commonly occurs in patients with underlying risk factors and at high doses, and the risk of arrhythmias in patients with a normal QTc may be somewhat overblown [8].
Central nervous system: Typical antipsychotics like haloperidol are famous for causing drug-induced movement disorders, also known as extrapyramidal symptoms. These include dystonia (continuous, often painful muscle spasms and contractions) and akathisia (motor restlessness). These reactions can be treated with anticholinergics like benzotropine or diphenhydramine. Haloperidol can also result in parkinsonian symptoms, characterized by rigidity and bradykinesia that responds to cessation of the drug. Perhaps the most troublesome extrapyramidal symptoms resulting from chronic use, tardive dyskinesia, are irregular facial movements such as grimacing and tongue smacking, which may be permanent. Haloperidol can also trigger neuroleptic malignant syndrome, a life-threatening neurologic disorder characterized by muscle rigidity, fever, and autonomic instability [1,9].
Cautions
QT prolongation: Haloperidol should be used with caution in patients with electrolyte abnormalities (hypokalemia, hypomagnesemia), hypothyroidism, concomitant use of QT-prolonging medications, familial long-QT syndrome, or other conditions which may predispose to QT prolongation. Risk of QT prolongation and torsades is greatest with intravenous administration. While behavioral disturbances in the ED are more commonly treated with an intramuscular route of administration, if intravenous administration is to be used, consider obtaining a baseline EKG before initiation of therapy. Consider cardiac monitoring if the patient has risk factors for QT prolongation [2].
Elderly patients: In patients 65 and older with dementia, antipsychotics such as haloperidol are considered high risk and potentially inappropriate medications on the Beers List due to increased risk of mortality. Antipsychotics should only be used for behavioral problems if alternative therapies have failed and patients behavior poses risk to self or others [10].
Dosing and adjustments
- Psychosis: Oral: 0.5 to 5 mg 2-3 times daily. Intramuscular: 2-5 mg, may redose as often as every 60 minutes, though every 4-8 hours is often adequate.
- Rapid management of agitation and violent behavior (off-label use): 2.5 to 5 mg intramuscular with some individuals requiring up to 10 mg.
- Chemotherapy-induced nausea and vomiting (off-label use): 0.5 to 1 mg oral/IV every 6 hours as needed.
- In geriatric populations, the dose should be reduced. For psychosis: 0.5 to 2 mg by mouth 2-3 times per day [1].
- For psychosis or agitation related to Alzheimer’s and other dementias (off-label use): 0.25 to 0.5 mg by mouth per day; slowly increased dose based on response and tolerability every 4 to 7 days in increments of 0.25 to 1 mg; usual maximum dose of 2 mg/day [1].
Special Considerations
Pregnancy category C: Haloperidol crosses the placenta, and adverse events have been reported in animal reproduction studies. Although haloperidol has not been proven to be a major human teratogen, there are concerns with use in pregnancy, and an association with limb abnormalities following first trimester exposure cannot be ruled out [11]. Use during third trimester is also problematic, and may cause extrapyramidal symptoms and withdrawal symptoms in newborns following delivery. Haloperidol is excreted in breast milk, and breast-feeding is not recommended [1].
Cost
- Haloperidol oral tablets (generic): 0.5 mg tablets (100): $32.551; 5 mg tablets (100): $102.38.
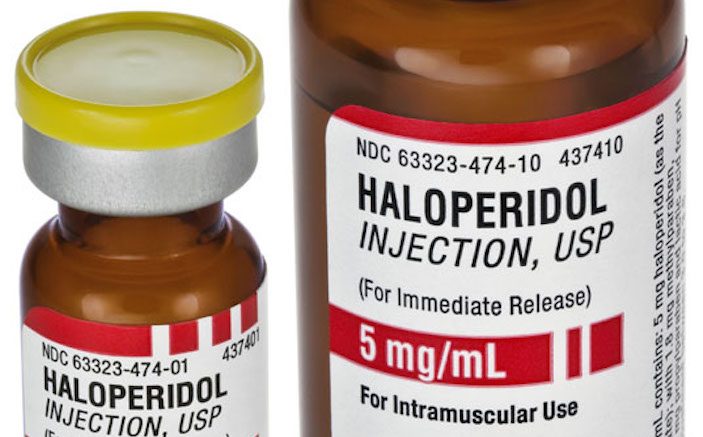
- Haloperidol decanoate solution for intramuscular injection (generic): 50 mg/mL (1mL): $7.63; 100 mg/mL (1 mL): $61.78.
- Haldol decanoate solution for intramuscular injection (brand name): 50 mg/mL (1mL): $104.49; 100 mg/mL (1 mL): $199.19.
REFERENCES
- Lexicomp. Haloperidol: Drug information. available at http://www.uptodate.com. accessed august 14, 2016. .
- Drugbank: Haloperidol. available at http://www.drugbank.ca/drugs/DB00502. accessed august 19, 2016. .
- Yildiz A, Sachs GS, Turgay A. Pharmacological management of agitation in emergency settings. Emerg Med J. 2003;20(4):339-346.
- Nobay F, Simon BC, Levitt MA, Dresden GM. A prospective, double‐blind, randomized trial of midazolam versus haloperidol versus lorazepam in the chemical restraint of violent and severely agitated patients. Acad Emerg Med. 2004;11(7):744-749.
- Clinton JE, Sterner S, Stelmachers Z, Ruiz E. Haloperidol for sedation of disruptive emergency patients. Ann Emerg Med. 1987;16(3):319-322.
- Honkaniemi J, Liimatainen S, Rainesalo S, Sulavuori S. Haloperidol in the acute treatment of migraine: A randomized, Double‐Blind, Placebo‐Controlled study. Headache: The Journal of Head and Face Pain. 2006;46(5):781-787.
- Granger B, Albu S. The haloperidol story. Annals of Clinical Psychiatry. 2005;17(3):137-140.
- Meyer‐Massetti C, Cheng CM, Sharpe BA, Meier CR, Guglielmo BJ. The FDA extended warning for intravenous haloperidol and torsades de pointes: How should institutions respond? Journal of hospital medicine. 2010;5(4):E8-E16.
- Lee A. Treatment of drug-induced dystonic reactions. Journal of the American College of Emergency Physicians. 1979;8(11):453-457.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American geriatrics society updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
- Armstrong C. ACOG guidelines on psychiatric medication use during pregnancy and lactation. American Family Physician. 2008:78(6):772-778.




2 Comments
Good review, but not distinguishing between the immediate-acting IM or IV Haldol and Haldol decanoate, which is the only parenteral form mentioned specifically in the article (in the cost section), may give rise to confusion.
Any special considerations with alcoholics?